The crowded environment inside a biofilm results in strong competition between biofilm dwelling cells for resources, including nutrients and space. The competition for available space on a surface is a unique aspect of the biofilm growth mode. Inevitably, competition for space involves mechanical interactions between individual biofilm cells and between biofilm clusters. On the other hand, the physical proximity between cells also invites collaboration: cells can share public goods such as extracellular polymers, but they also have to evolve mechanisms to defend against cheater cells. We are generally interested in what intrinsic genetic and mechanical mechanisms and what external factors control the social ecology inside such microbial communities. Below are few examples.
We have shown that the compact V. cholerae biofilms are able to outcompete weaker, less dense mutant biofilms that lack cell-cell connection protein RbmA. Although the mutant biofilms occupy a larger volume in the beginning, eventually they are mechanically shoved away by the stronger, more compact biofilms in which individual cells are connected (Yan et al. 2016).
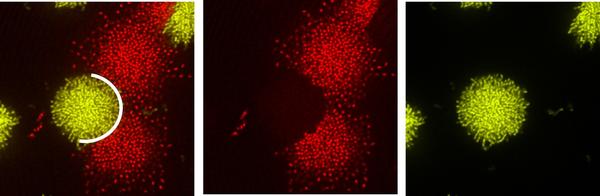
Figure 1: Collision between rugose V. cholerae biofilms (yellow) and mutant biofilms lacking cell-cell connection (red). The mutant biofilm clusters are significantly deformed, whereas the biofilm from the parent strain is not.
We also discovered that matrix-producing cells are able to take advantage of the osmotic contrast generated by the extracellular polymers to exclude and outcompete non-producer cells and invading cells (Yan et al. 2018).
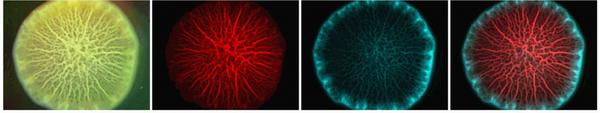
Figure 2: Bright field and fluorescence images of a 2-day-old biofilm on agar starting from a 1:1 mixture of the matrix producing strain (red) and the nonproducing strain (cyan).
Finally, we found that environmental fluctuations encountered by bacteria have a strong influence on the fitness of cells with different biofilm forming strategies. Wild type V. cholerae cells adopt a flexible strategy that allows a fast transition from the planktonic state into the biofilm forming state, and vice versa, which presumably maximizes their survival in nature and during infections (Yan et al. 2017).